肌肉减少症对全身治疗的胃肠癌患者急性化疗毒性的影响
点评:郑振东 郑振东 沈阳军区总医院肿瘤科
翻译:关欣
来源:肿瘤资讯

关欣硕士研究生
锦州医科大学肿瘤专业硕士研究生;参与国家自然科学基金和辽宁省自然基金2项,参编专著1部。主要研究方向为消化道肿瘤营养与代谢调节治疗。导师:沈阳军区总医院肿瘤科郑振东主任。
背景
该研究目的是评估肌肉减少症对全身化疗胃肠道癌症(GISC)患者化疗耐受性的影响。
方法
纳入既往未接受化疗或放疗的GISC患者。 在化疗的第一个周期之前通过生物阻抗评估所有的肌肉质量,并且根据第一生物阻抗评估结果重新评估2-3个化疗周期后具有正常肌肉质量的患者。在肌肉减少症和非肌肉减少症患者中比较急性毒性。
结果
在第一次评估中共有116名患有肌肉减少症的患者被纳入研究人群。 中位年龄为57岁(最小值:26岁最大值:76岁),其中2/3为男性(67.6%)。 原发肿瘤位置为结肠直肠癌(51.8%),胃癌(44.1%),食管癌(4.1%)。 化疗为佐剂(46.9%),新辅助(22.1%)和姑息治疗(31%)。 在第二次生物阻抗评估中,有36例(31%)患者检测到肌肉减少症。 pts的基础体重指数(BMI)评估显示,与非肌肉减少症患者相比,肌肉减少症患者肥胖和超重患者的发生率显着降低(44.4%vs 68.8%,p <0.05)。 在第二次生物阻抗测量时,肌肉减少症和非肌肉减少症患者的BMI之间没有显着差异。 肌肉减少症和非肌肉减少症组的化疗毒性率如表所示:
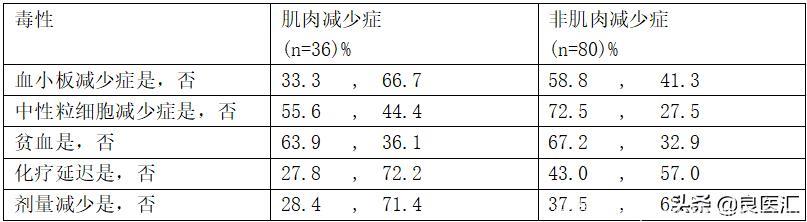
结论
在GISC患者诊断时非肌肉减少症,化疗2-3周后BMI保持不变。 然而,其中1/3患者的肌肉减少了。 发生肌肉减少症的患者化疗相关毒性的发生率较低。 应进一步调查造成这种差异的原因。化学治疗剂亲脂性和亲水性的不同可能是解释之一。
专家点评

郑振东医学博士、研究生导师、教授
沈阳军区总医院肿瘤科主任,中国医科大学、大连医科大学、沈阳药科大学、锦州医科大学硕士研究生导师。从事恶性肿瘤综合治疗的临床及科研工作近20年,擅长乳腺、呼吸系统、消化系统、泌尿生殖系统等部位常见及各种罕见恶性肿瘤的科普宣教、预防、诊断及综合治疗。常年致力于各种实体肿瘤的精准诊疗和临床转化研究,擅长乳腺、呼吸道、胃肠道等恶性肿瘤的预防、诊断及治疗。主持科研立项9项,共计70余万元;获得省部级以上科研奖励10项,发表中文核心期刊近50篇,SCI收录 20篇,总影响因子42.79分;担任辽宁省细胞生物学学会肿瘤精准治疗与大数据管理分会主任委员、辽宁省抗癌协会肿瘤标志专业委员会副主任委员、辽宁省自然科学基金项目评审专家等40余项学术兼职。
“肌肉减少症”一词由美国营养学家罗森伯格首次提出,用于形容因衰老引起的肌肉质量减少[1]。肌肉减少症是一种综合征,其特征是骨骼肌质量(SMM)和强度的进行性和全身性丧失。在临床实践中,将肌肉减少症可分为“原发性”和“继发性”类型:除了老化本身之外,没有其他原因可以被定义为“原发性”(或与年龄相关)。相反,当肌肉减少症源于一种或多种病因时,可将其定义为“继发性”。继发性肌肉减少症又区分为:“活动相关的肌肉减少症”,源于固定或久坐的生活方式; “与疾病相关的肌肉减少症”,与晚期器官衰竭,炎症性疾病,恶性肿瘤或内分泌疾病有关; 和“营养相关的肌肉减少症”,与营养不良,吸收不良或胃肠道疾病有关[2]。据报道,肌肉减少症与许多恶性肿瘤的预后不良有关,包括肝细胞癌,肺癌,膀胱癌,胰腺癌,黑色素瘤和结肠直肠癌[3]。该研究评估了肌肉减少症对全身化疗的消化道肿瘤患者化疗耐受性的影响,研究发现非肌肉减少症患者化疗后的BMI较化疗前没有显着差异,而其中1/3患者发生了肌肉减少,并且发生肌肉减少症的患者化疗相关毒性的发生率较低。这一结果表明化疗可能会引起肿瘤患者肌肉减少,而肌肉减少症对胃肠道肿瘤患者化疗耐受性有较为积极的影响。这与先前的一些研究结果不同,之前研究发现骨骼肌质量丧失和预后不良及化疗毒性之间存在密切联系,揭示肌肉质量的减少会增加发生化疗毒性的风险,降低转移性结直肠癌患者的生存率[4,5],而该研究得出相反的结论。这可能与入组的人群选择的偏差、确定骨骼肌质量的方法或治疗方案的异质性有关。探索该现象发生的原因及机制,为肌肉减少症与化疗不良反应耐受性之间的关系做出更准确可靠的阐明,为化疗不良反应的防治提供可靠的理论依据。
参考文献
1. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S.
2. Ilaria Liguori, Gennaro Russo, Luisa Aran, et al.Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018; 13: 913–927.
3. Naruji Kugimiya, Eijiro Harada, et al. Loss of skeletal muscle mass after curative gastrectomy is a poor prognostic factor. Oncol Lett. 2018; 16(1): 1341–1347.
4.Miyamoto Y, Baba Y, Sakamoto Y, et al. Negative impact of skeletal muscle loss after systemic chemotherapy in patients with unresectable colorectal cancer. PLoS One. 2015; 10(6): e0129742.
5.Barret M, Antoun S, Dalban C, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer 2014;66:583–589.
附原文:
The effect of sarcopenia on acute chemotherapy toxicity in gastrointestinal cancer patients undergoing systemic therapy (Abstract 1211P)
Background: We aimed to evaluate the effect of sarcopenia on tolerability of chemotherapy during systemic treatment of pts with gastrointestinal system cancer (GISC).
Methods: Patients with GISC who had not previously received chemotherapy or radiotherapy were included. All of the pts muscle mass was evaluated by bioimpedance prior to the first cycle of chemotherapy, and pts who had normal muscle mass according to first bioimpedance evaluation were re-evaluated after 2-3 cycles of chemotherapy. The acute toxicities were compared in sarcopenic and non-sarcopenic pts. To define the sarcopenia results of a Turkish validation study was used (sarcopenia for male < 0.05). At the time of the second bio impedance measurement, there was no significant difference between BMI of sarcopenic and nonsarcopenic pts. The rate of chemotherapy toxicities of the sarcopenic and non-sarcopenic group is shown in the table:
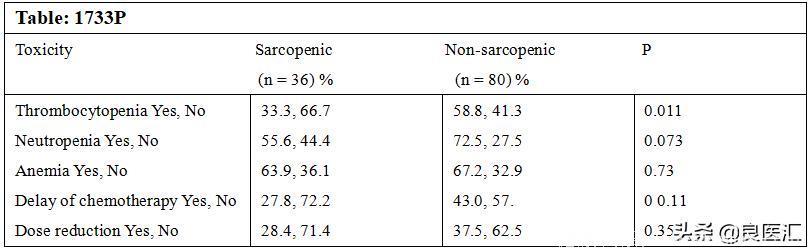
Results:A total 116 pts who had nosarcopenia in the first evaluation were included in the study population. Median age was 57 (min:26-max:76) and 2/3 of them were male (67.6%). Primary tumor locations were colorectal (51.8%), gastric (44.1%), esophagus (4.1%). Chemotherapy was given as adjuvant (46.9%), neoadjuvant (22.1%) and palliative (31%). In the second bioimpedance evaluation sarcopenia was detected in 36 (31%) pts. Basal body-mass index (BMI) evaluation of pts showed that rate of obese and overweight pts was significantly lower in sarcopenic pts compared to non-sarcopenic pts (44.4% vs 68.8%, p<0.05). At the time of thesecond bioimpedance measurement, there was no significant difference between BMI of sarcopenic and nonsarcopenic pts. The rate of chemotherapy toxicities of the sarcopenic and non-sarcopenic group is shown in the table:
Conclusions: In GISC pts non-sarcopenic at time of diagnosis, BMI remains unchanged after 2-3 cycles of chemotherapy. However, muscle loss had developed in 1/3 of them. Pts who developed sarcopenia had lower rates of chemotherapy-associated toxicity. The reasons for this difference should be further investigated. However, the different lipophilic and hydrophilic properties of chemotherapy agents might be one of possible explanation.
版权声明
版权属肿瘤资讯所有。欢迎个人转发分享,其他任何媒体、网站如需转载或引用本网版权所有内容,须获得授权,且在醒目位置处注明“转自:良医汇-肿瘤医生APP”。